
GE P-Port eCoil Interface Device
Part of our success comes from our ability to present solutions via medical device development, so that diagnosticians can work from higher quality MRI images.
Such is the case in the research and development of our newest product, the GE 1.5T P-Port Interface Device. This connector device clears the way for the latest GE MRI imaging scanners to connect to the MR Endorectal Coil now securely. The eCoil interfaces also with Siemens, and Phillips MRI scanners around the world.

The eCoil enhances prostate MRI imaging quality by providing a high signal-to-noise ratio (SNR), which enables the sensitivity needed for multi-parametric and spectroscopy imaging on 1.5 and 3.0T MRI scanners. The small field of vision and high spatial resolution images from the eCoil can assist the radiologist by delivering improved sensitivity and specificity in diagnosis and aid in treatment planning.
The new P-Port connector is perfect for newer model GE imaging equipment, GE 1.5T Artist and GE Voyager scanners. The new connector enables these GE devices to now utilize eCoils and obtain better image quality for prostate imaging. The Interface Device with HD connector is compatible with older generations of GE MRI scanners.
Interested in learning how the P-Port Connection can maximize your GE 1.5T scanner?
Connecting to Unmatched Imaging
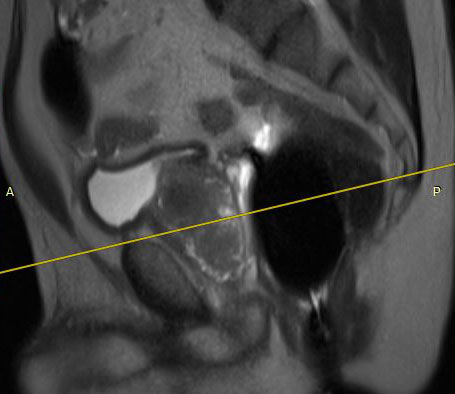
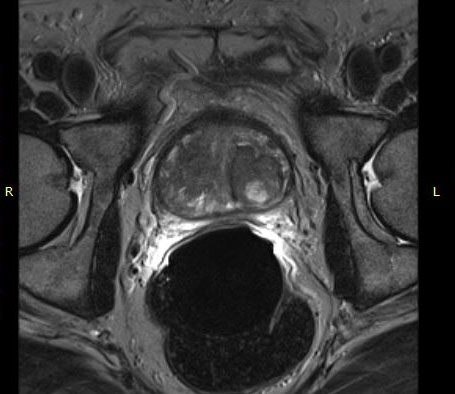
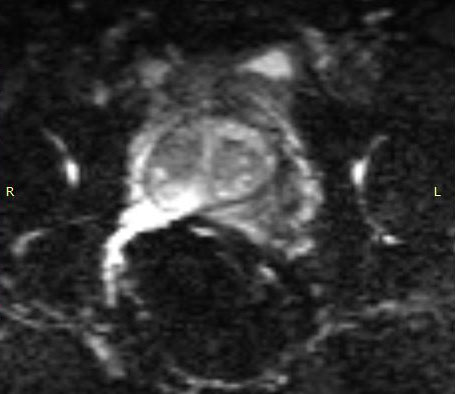
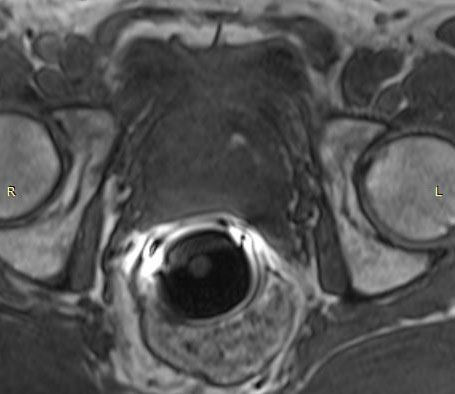
The importance of enabling the thousands of diagnosticians who use GE imaging equipment to now work with the eCoil cannot be overstated. The eCoil presents substantial advantages with multi-parametric imaging, especially Diffusion Weighted Imaging. The eCoil’s high SNR is needed by the DWI to produce high-quality prostate imaging. The eCoil provides higher SNR, higher resolution and assists with better lesion detection, better staging and better mpMRI than existing imaging techniques.
And, higher quality imaging contributes to a more confident diagnosis, which enables a successful treatment plan.
Promises Made. Promises Kept.
When DxTx Medical acquired the eCoil product line from Bayer in August of 2019, one of the primary goals was to invest in upgrading interface device capabilities for newer platforms of MRI scanners.
Within months of the acquisition, DxTx completed MDSAP certification to ISO 13485:2016, including FDA, TGA, and Health Canada requirements, as well as product certification to MDD 93/42EEC Annex II Full Quality Assurance. Securing these certifications establishes our global licensing and distribution infrastructure. That assures end-users have the capability to provide high quality products and services to their patients by using DxTx Medical equipment.
This latest release of the GE 1.5T Interface Device continues to deliver on the DxTx Medical objective to upgrade interface device capabilities. The connector will assist clinicians with greater access to scanners by providing better tools that gives them increased image quality, so that they can offer the best possible diagnosis, staging and treatment.


